Quantum dots are a class of “nanocrystals” with a very small diameter of often only one or a few nanometers. Because of their special properties, they are used in optical or electronic applications, e.g. in photovoltaics for solar cells. Quantum dots are also being tested in the medical field, as the tiny particles are able to visualise tumours in tissue, for example, through their optical properties.

QLED TV © Sergey Ilin / fotolia.com
How could I get in touch with it?
At present, the consumer hardly comes into contact with quantum dots, as they are of the greatest importance for research. Their use in everyday products is limited to QLED TVs and displays from a few manufacturers. Because the quantum dots are inside the electronic device the consumer does not come into direct contact with quantum dots.
Is there any risk from this material to humans and the environment?
Since consumers do not come into contact with quantum dots, the following applies only to employees of research laboratories: When quantum dots are swallowed, the stomach acid often peels off the coating of the particles so that the inner material is exposed. Quantum dots are only absorbed through the skin if it has already been damaged. There the particles can also cause inflammation. After absorption into the body, the quantum dots accumulate in the liver and spleen as is usual for comparable particulate materials. As they are relatively stable, they can remain in the organs for a long time. All studies on the uptake, distribution and passage through the blood-brain barrier were carried out with animals, mostly after intravenous administration, which is why they have only limited significance for a risk to humans in normal life.
Conclusion
Quantum dots are of great scientific interest, but are currently only used sporadically in consumer-related products. Since they may also contain toxic elements such as cadmium, their use in humans will be limited in the future.
Properties and Applications
Nanocrystals in which so-called quantum effects occur due to their extremely small diameter (in the range of a few nanometers) are called quantum dots. These do not consist of a uniform material, but describe an entire class of materials. Quantum effects cause extremely interesting optical, magnetic and electronic properties in nanocrystals. For example, they can shine (fluoresce) with the aid of light, supply electricity very efficiently or serve as a super-small memory or processor elements in IT.
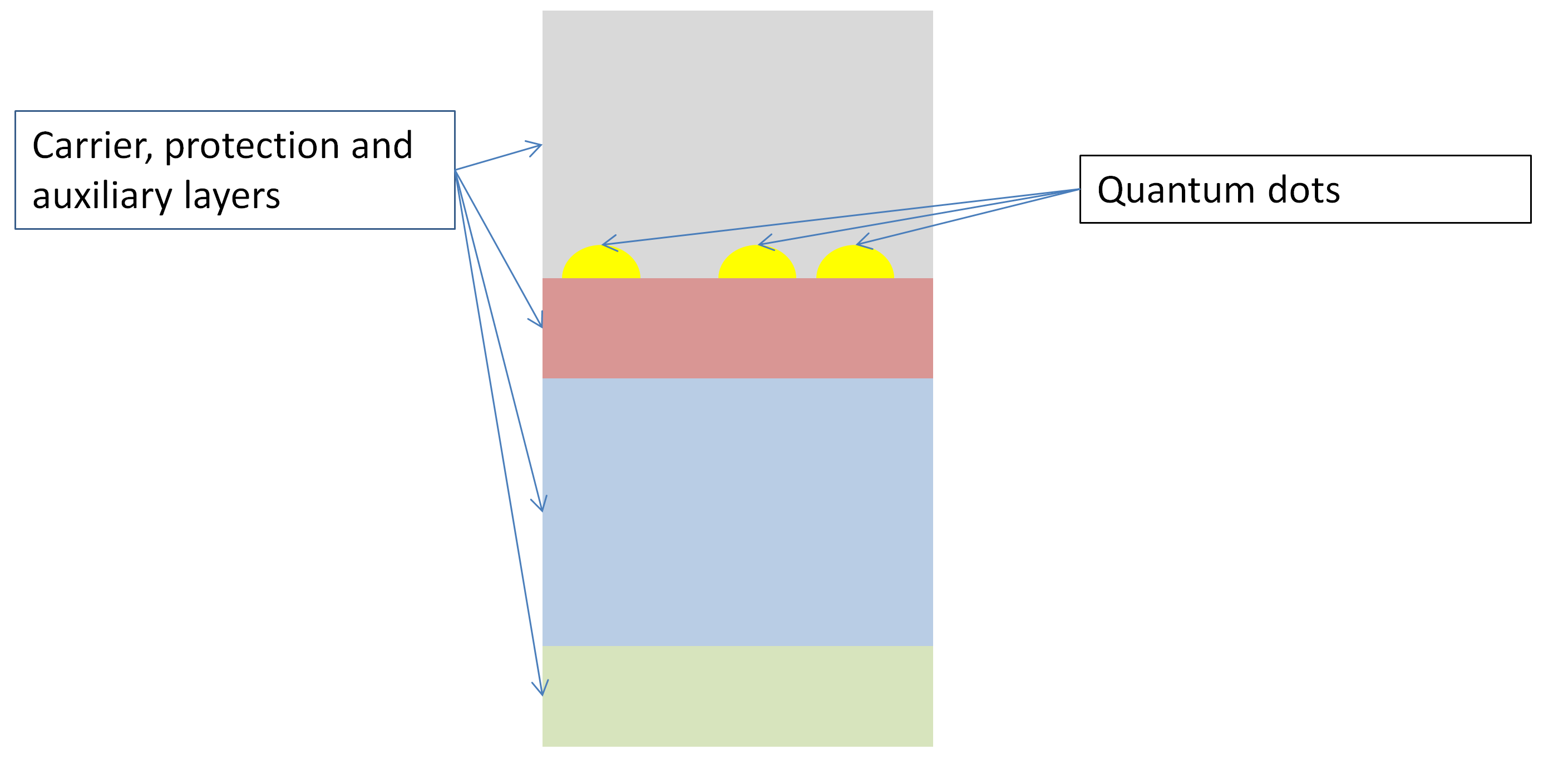
Schemtic overview of the set-up of Epitactic Quantum Dots © C. Steinbach
With a size of about 1-100 nm, quantum dots consist mostly of semiconductor materials. They are made of either one or different materials, which follow a construction principle of core and shell. Often different materials are used for the core and shell, whereby several coating layers are also possible. Both the electronic and optical properties of the quantum dots can be precisely adjusted with these so-called core-shell structures, which make them very interesting for a number of applications.
For free metallic quantum dots there is a theoretical risk that they can self-ignite because of their large surface area. However, as they are usually only processed embedded in liquids or plastics and used in very small quantities, spontaneous combustion is very unlikely.
There are three main types of quantum dots:
- III-V-semiconductors: made of elements of main group III of the periodic table of the elements (boron, aluminium, gallium, indium) and main group V (nitrogen, phosphorus, arsenic, antimony, bismuth)
- II-VI- semiconductors: made of elements of transition metal group II (zinc, cadmium) and main group VI (oxygen, sulphur, selenium, tellurium)
- Silicon (Si), the standard material of the semiconductor and chip industry
The best-known representative of III-V semiconductors is gallium arsenide (GaAs). In the field of optical data processing, it serves primarily as a light source and is also used as an amplification medium in lasers. However, gallium arsenide appears to be restricted to special applications and does not compete with silicon in the semiconductor industry.
The most prominent representatives of the II-VI semiconductor quantum dots are cadmium selenide (CdSe) and cadmium telluride (CdTe). Zinc oxide (ZnO), which is already widely used in the form of micro and nanoparticles, is also increasingly being used as a material in quantum dots. Thanks to their outstanding fluorescence properties, II-VI semiconductor materials are used in electronics, photonics, photovoltaics and biomedicine. Cadmium selenide-based quantum dots are preferably found in lighting applications and displays based on quantum dot LEDs.
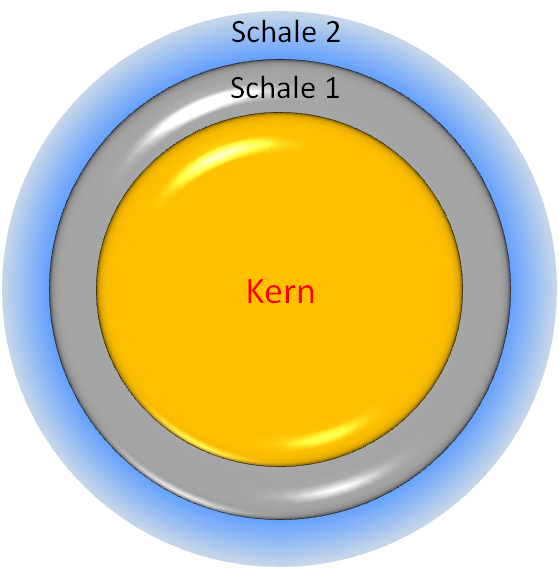
Schematic structure of a free core-shell quantum dot © C. Steinbach
In thin-film solar cells, the use of cadmium telluride-based quantum dots is currently being tested. They promise a significant increase in efficiency. However, since these quantum dot materials contain toxic cadmium, further research is being conducted into alternatives. II-VI quantum dots are used as biomarkers for the detection of biomolecules in medical samples.
Silicon quantum dots are currently not as advanced as III-V and II-VI semiconductors. However, they promise great potential for integration into current silicon electronics, e.g. as a component of optical chips, processors, optical sensors or in photovoltaics for achieving major advances in efficiency. Due to the current high price, such silicon materials are mainly used in the space industry.
Quantum dots are still a major topic for research. They are currently only used sporadically in consumer-oriented products. Many concepts and effects need to be examined in more detail.
Fabrication

QD ©Leo / Fotolia.com
Quantum dots were first discovered in the 1980s. Today's production methods, including chemical processes in solutions, photolithography or molecular beam epitaxy, vary depending on the starting material.
The starting material for silicon quantum dots is silicon dioxide. Further silicon ions are introduced into a corresponding matrix of silicon dioxide and then heated at high temperatures for a longer period of time until the desired nanocrystals form.
In electron beam lithography, quantum dots are "written" onto an appropriate substrate via an electron beam and then exposed by a suitable etching process. In a simplified description of the complex process a special lacquer is applied to a surface, which contains the components for the generation of the desired quantum dots. The spot-like electron beam converts the components into quantum dots at the very small points, where it hits the lacquer surface. The excess paint residues are then removed. Disadvantages of this method are poor reproducibility and high effort.
Molecular beam epitaxy is used to produce quantum dots from III-V semiconductors such as gallium arsenide. Here, the two metals gallium and arsenic are vaporized simultaneously and then shot at a surface. Alternatively, they can also be produced from organometallic compounds by so-called gas phase deposition. In this procedure the quantum dots are created directly on the substrate required for the respective application.
Finally, colloidal quantum dots can also be produced from III-V semiconductors and II-VI semiconductors using chemical processes in solutions, usually for use in biological media. The quantum dots developed in the mid-1990s, for example, consisted of a core of cadmium selenide with a shell of zinc sulfide. First, cadmium selenide nanocrystals are precipitated from a cadmium salt solution with selenide anions. Using the same principle, a zinc sulfide coating is then grown on these nanocrystals.
During the manufacturing process, workers could ingest quantum dots through the lungs, swallow them or come into contact with them via the skin. The biological effect of quantum dots is controversially discussed in the current literature. Studies have shown that quantum dots can be distributed in the organism and possibly accumulate in different organs and tissues.
General Hazards
Due to their extraordinary optical properties, quantum dots have many advantages over the use of organic fluorescent dyes. They are primarily qualified for biological labelling methods or applications in clinical diagnostics . Moreover, specific drugs or other target molecules (e.g. antibodies or peptides) can be attached to the surfaces of quantum dots. With such a vehicle drugs may be targeted very specifically to certain cells and tissues to act there directly . Such an effect can be achieved only after intravenous injection. Several studies investigated distribution, excretion, metabolism and toxicity of quantum dots after this form of application . Furthermore, skin penetration and the behaviour of quantum dots within the gastrointestinal tract after swallowing have been analysed .
Studies on Living Organisms - in vivo
The distribution of various quantum dots (QDs) in the body of animals has been tested by intravenous injection into mice and rats and has been analysed after different time points of treatment . Some examples will be discussed below in more detail.
After the injection into the tail vein of mice all QDs distribute rapidly within the blood circulation even though various coatings have been used. Within one hour nearly all QDs had left the blood stream and could be detected depending on their coatings within the liver, skin or the bone marrow . Only one very specific coating could prevent the fast elimination from the blood. Even after 133 days of observation the QDs could still be detected due to their strong fluorescence within the bodies of the animals. The stable high fluorescence signal indicates no degradation of the QDs within the organisms. Moreover, during the whole period of the experiment no cell death or acute toxicity could be detected in the surrounding tissues . Changing the coating may result in degradation of the QDs as has been shown in other experiments in which a loss of luminance could be observed . Probably coatings with larger molecules (high molecular weight) protect much better against degradation as compared to smaller molecules with a low molecular weight .
Within a 10-days short term study in rats it could be demonstrated that QDs depending on their coating could be detected within the liver and the spleen and to a lesser extent within the lung tissue and the kidneys . During the whole observation time the QDs were stable (no degradation) and were not excreted via urine or faeces. It has been discussed that the used QDs are too large to be excreted via the kidneys .
In agreement with this a longer study (28 days) showed in mice that QD accumulate in liver, spleen and kidneys without being excreted . Treatment and observation of mice over a period of two years (!) after injection of QD resulted in the following data: shortly after injection the QD could be detected in liver, spleen, lymph and the bone marrow. The fluorescence signal weakened within 5 days in the liver but stayed constant for 6 months within the bone marrow before decreasing there as well. Overall the signal could be detected over the whole period of 2 years within the lymph, no degradation took place and also no toxicity could be detected after this time of treatment .
Studies Outside of Organisms - in vitro
Quantum dots (QDs) can be produced from a variety of different materials and they often have different coatings, thus, it cannot be expected that they provoke the same biological response in different cell lines or animals. In vitro toxicity studies have been performed in a variety of different cell types with an even bigger set of different or differently modified QDs. As a general rule physico-chemical properties such as size, core and shell material, stability of the shell material, type of coating, the production process as well as the surface charge of QDs can influence their biological effects. Furthermore, the cell type(s) in which toxicological measurements are being performed as well as the analysed concentration range have a major effect on the obtained results .
The QD core can be protected from degradation and release of e.g. toxic Cd2+ ions by various coatings . The stability of the QD thus largely depends on the stability of its shell and/or coating material. How stable QDs are inside living cells or even organisms (see section “exposure") strongly depends on their intracellular localisation (see section ”behaviour of uptake in somatic cells” ) .
In contrast to the protective effect of such coatings the material used to shield the core could itself affect cellular viability . The coating material should thus be chosen very carefully. Furthermore excessive coating will increase the size or the hydrodynamic diameter (HD) of the QDs. This parameter becomes very important in animals during the process of excretion (see section “behaviour inside the body”).
Besides the release of Cd2+ ions the cytotoxicity of QDs has also been explained by the induction of reactive oxygen species (ROS) . In high amounts these free radicals can induce damage to intracellular proteins, lipids and nucleic acids which can subsequently lead to cell death . The subcellular distribution of Cadmium-Telluride (CdTe) -QDs has been shown to be size-dependent in two different cell types. While smaller QDs (2 nm) localise to the nuclear compartment larger ones (5 nm) localise to the cytosol . Furthermore the same study reports that cytotoxicity is more pronounced with positively-charged smaller QDs compared to equally-charged larger QDs.
In addition Guo and colleagues report that positively-charged QDs lead to significant cell death in liver cells indicating that surface charge of QDs has some effect on cell viability. In contrast to some of the results described above, another group of scientists shows – using again two other cell types – that the size of QDs does not influence their uptake and distribution. Moreover, no toxic effects of the QDs can be observed for up to ten days .
So far it is not clear if the QD has to enter a cell in order to exhibit negative effects or if an exterior contact between the QD and the cell is sufficient. The cytotoxicity of QDs has been described to correlate much better with intracellular concentrations of QDs than with extracellular exposure levels . Furthermore the researchers conclude that this toxicity is mainly due to degradation of the particles and with that the release of toxic Cd2+ ions. This observation correlates very well with results discussed above. On the contrary other scientists could also show that the mere precipitation of QDs on the cell surface is enough to impair cell viability, though in a different manner compared to ingested particles .
In addition to cytotoxicity in general, damaging effects towards the genetic material (DNA) of a cell was assessed in several studies . DNA strand breaks could be observed with and without exposure to light maybe involving reactive oxygen species . Moreover the core or core-shell material respectively seems to determine the severity of DNA damage. While Cadmium-Telluride (CdTe) core particles showed the highest DNA damaging activity, CdTe/SiO2 core-shell particles were less potent and Manganese:Zinc-Selenide (Mn:ZnSe) particles induced almost no DNA damage .
Currently, there are no data are available regarding an environmental exposure of quantum dots.
Only a few studies are currently available on the absorption of quantum dots into the body. Various enveloping and coating materials protect the core of the quantum dots and thus prevent their release.
Uptake via the Skin - Dermal Uptake
The penetration of Quantum Dots through intact skin was analysed in a porcine skin ex vivo perfusion model . In this experimental set-up, the porcine skin was perfused with blood containing different concentrations of Quantum Dots. Depending on the surface modification (either carboxyl (COOH) or PEG) of the Quantum Dots, a larger or lesser amount could be found in skin capillaries, respectively, indicating that uptake could occur from the blood to the skin tissue itself . This confirms earlier work from the same group showing in vitro that COOH-coated Quantum Dots are taken up in greater quantities into human keratinocytes (skin cells) than PEG-coated ones .
The same ex vivo model as well as skin cells (keratinocytes, in vitro) were used to assess effects of topically applied Quantum Dots . PEG-coated Quantum Dots were found to only penetrate the uppermost stratum corneum (horned layer of the skin) which consists of dead cells. However the viability of living skin cells (keratinocytes) in vitro decreased with increasing concentrations of the same QDs .
The effects of Quantum Dots on sun-injured skin were assessed using UV-irradiated mouse skin in an in vivo model .It could be shown that after UV exposure a greater amount of Quantum Dots was found in deeper layers of the skin compared to uninjured skin were almost no penetration took place. The authors conclude that amount and depth of Quantum Dots penetration largely depends on the condition of the skin and the characteristics of the Quantum Dots (like e.g. size and surface chemistry) .
Furthermore, comparing different skin injury types (“tape stripping, which removes the horned layer only; acetone treatment, dermabrasion, flexing) in mouse and rat models revealed that only dermabraded skin was leaky for Quantum Dots .Particles were applied to the skin of injured animals as well as uninjured control animals. Approximately 2% of the applied dose of cadmium (resulting from the Quantum Dots' Cadmium-Selenide cores) could be detected in both the lymph and the liver of dermabraded mice. These results suggest that if skin is reasonably damaged it is possible for Quantum Dots to penetrate into deep layers of the skin and even distribute around the body via the bloodstream – at least in this particular mouse model and for this type of Quantum Dots.
In summary, it can be concluded from these data that only a very small proportion of superficially administered quantum dots can penetrate the intact skin. However, if the skin is damaged and thus facilitates the penetration of the quantum dots, it may be possible that the application of Quantum Dots may impact or impair the cells of deeper skin layers and trigger inflammatory reactions.
Uptake via the Gastro-Intestinal Tract
Biomedical applications for Quantum Dots, including in vivo imaging or drug delivery, require their targeting to the correct position in the body (see also the article on “behaviour inside the body”). This targeting can be achieved by specific coatings. However these coatings have been shown to degrade in acidic environments thereby releasing toxic cadmium ions (see also the article on “exposure”). Degradation could thus occur either in intracellular compartments like lysosomes or in organs like the stomach. Therefore administration of Quantum Dots is largely restricted to intravenous injection. Nevertheless a very recent report addresses the biodistribution and stability of Quantum Dots in the digestive tract of the mouse after oral administration .
Scientists introduced a new surface coating for core-shell-shell Quantum Dots (CdSe-CdS-ZnS) that consists of a combination of polythiol-ligands and a silica shell. This modification renders these particles resistant to strong acidic solutions both in vitro and in vivo in the digestive tract of mice after oral administration . The authors conclude, that the new coating offers the possibility to use Quantum Dots for the study of in vivo processes within the digestive system.
Quantum Dots may consist of toxic metal compounds such as Cadmium-Telluride and Cadmium-Selenide. Toxic effects of Quantum Dots have been described due to the leaching of metal compounds. The coating of Quantum Dots also affects their toxicity. Numerous surface modifications were developed to prevent the leaching of toxic metals. Also the light quality and quantity play a role for the effect, UV-B irradiation toxifies the particles.
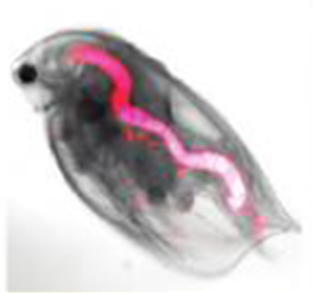
Water flea with ingested fluorescent quantum dots (pink colour). © Lewinski et al., 2010.
Different amounts of Quantum Dots with different coatings are ingested by water fleas; accordingly the excretion differs depending on the type of coating . The size was found to have no effect on the uptake . For various bacteria and water fleas, certain groups of Quantum Dots are toxic, although here, too, the type of coating and UV-light has an influence on the strength of the effect . The more heavy metals leach out, the greater the toxic effect . In the presence of environmentally relevant compounds binding to heavy metals, the toxic effects are reduced . Some Quantum Dots are able to slow down the microbial decomposition of dead organic materials in the environment i.e. they inhibit the activity of the bacteria.
Certain groups of Quantum Dots suspended in aqueous solutions proved to be toxic to algae . There was an inhibition of the photosynthetic performance of algae, i.e. the use of light for nutrition was limited.An uptake of the particles into algal cells was not observed. Instead, the particles adhered to the algal cell wall. When Quantum Dot-exposed algae were fed to water fleas, the Quantum Dots could be detected in the fleas. Hence, a transfer of Quantum Dots through the food chain from lower to higher organisms takes place .To diatoms, cadmium-containing quantum dots were cytotoxic depending on the release of the cadmium ions . This toxicity can be reduced by a surface coating, which prevents the release of cadmium ions.
In rainbow trout, the exposure to certain groups pf Quantum Dots had a negative influence on the immune system . Liver cells of rainbow trout were also damaged, depending on the release of cadmium . In zebrafish embryos, negative effects were observed. These were due to the various metal components of the Quantum Dots, which dissolve in small amounts, but also partly to the type of surface coating .
In summary, intact Quantum Dots, in particular those that do not emit toxic metal ions, are considered to be of low toxicity. Generally, for assessment of the toxicity of Quantum Dots, the type of surface coating has to be considered. With an extended persistence in the environment, increased release of toxic metals from heavy metal-containing quantum dots is associated, which have a toxic effect on organisms in the environment. However, a number of organic compounds in the environment, provided they are present, may bind the metal ions.
The uptake behaviour in cells depends on the coating of the quantum dots.
Behaviour inside the body
The longer cadmium-based quantum dots reside in the body, the greater is the chance of coating degeneration, subsequent leakage of cadmium ions and thus the induction of toxicity at sides of deposition.
Therefore, scientists systematically analysed the renal excretion of cadmium-based quantum dots in various sizes and surface modifications in a rat model. With increasing particles size the renal clearance rate of the quantum dots was drastically reduced and the larger nanoparticles were found in the liver, lung, and spleen instead. Consequently, the hydrodynamic diameter of the quantum dots used in biomedical applications should not exceed 5.5 nanometres for rapid and efficient urinary excretion. Binding of serum proteins may also result in an increase in hydrodynamic diameter up to 15 nm, as they preferentially bind to positively or negatively charged quantum dots. This behaviour can be avoided by using a suitable coating .
In conclusion, the production of quantum dots suitable for in vivo applications is complex and requires consideration of various other aspects in addition to the desired functionalities. On the one hand, the quantum dots with their respective coating should be as stable as possible to prevent leakage of cadmium ions. But on the other hand, a quick an efficient clearance of the nanoparticles from the body can only be ensured for quantum dots smaller than 5 nanometres, which is virtually impossible with currently used coatings.
Behaviour at the Blood-Brain Barrier
It is very challenging to deliver drugs to the brain because the blood brain barrier functions as a gatekeeper guarding the brain from exogenous substances.
In a recent study bioconjugated Quantum Dots were injected intraperitoneally (meaning into the body cavity) into mice and their distribution in the body was investigated . Scientists found, that the Quantum Dots reached the blood circulation and mainly accumulated within the spleen, the liver and the kidney (correlating very well with other studies). However, a very small but still considerable amount ended up in the brain. Although most of the particles resided in blood vessels of the liver, the kidney and the brain, a few reached the brain tissue.
Behaviour of uptake in somatic cells
Non-functionalised quantum dots are only equipped with a basic coating that does not serve any additional biological function. Further details on this topic can be found in the article "Exposure - Studies Outside of Organisms - in vitro". Such non-functionalised quantum dots usually enter cells via endocytosis and most often end up in the cytoplasm. Certain types of quantum dots could also be detected in the cell nucleus. Furthermore, different coatings of non-functionalised quantum dots seem to influence their uptake behaviour. The more biocompatible (thus non-toxic) the coating renders the quantum dots, the less cellular uptake occurs .
Quantum dots can also be functionalised by attaching specific biomolecules like peptides or antibodies to the surface of the nanoparticles. Such (bio)molecules are known as functional groups or moieties (parts or portions). The mode of entry into the cells as well as the intracellular localisation of certain groups of quantum dots strongly depends on the respective choice of functional moieties attached to the quantum dots surface
Various imaging techniques make use of this behaviour. Specific staining of the plasma membrane or various intracellular organelles can be realised using quantum dots labelled with antibodies. Furthermore, selected protein markers such as immunoglobulin G can specifically identify and visualize breast cancer cells .
In summary, varying the coating of quantum dots can be used to selectively control the uptake behaviour of the nanoparticles into the cell. In addition, quantum dots equipped with specific biomolecules can be used to label and visualise specific cell types or intracellular structures.
Quantum Dots often consist of a heavy metal core with a coating of organic substances which are intended to stabilise the particles. Because of their production and use, they are present almost only in aqueous suspensions; hence, the studies deal exclusively with the behaviour in water. The behaviour of Quantum Dots in the aquatic environment is largely determined by the solubility of the metal components.
Under certain conditions this coating can be lost, which can lead to leaching of toxic heavy metals. Part of these dissolved metals could be bound by numerous compounds present in the environment (dissolved organic materials, proteins) and are therefore no longer available for organisms .
Depending on the type of organic coating, agglomeration and sedimentation of the QDs occur under specific environmental conditions . Quantum Dots interact with compounds present in the environment (dissolved organic materials, alginates, metal ions), which either bind to the particles, change their agglomeration and sedimentation behaviour, or restrict the fluorescence of the particles . Quantum Dots can slow down the microbial decomposition of dead organic materials in the environment .
In general, when present in the environment over longer periods of time, the particles seem to dissolve and release e.g. Cadmium ions .
 >
>